
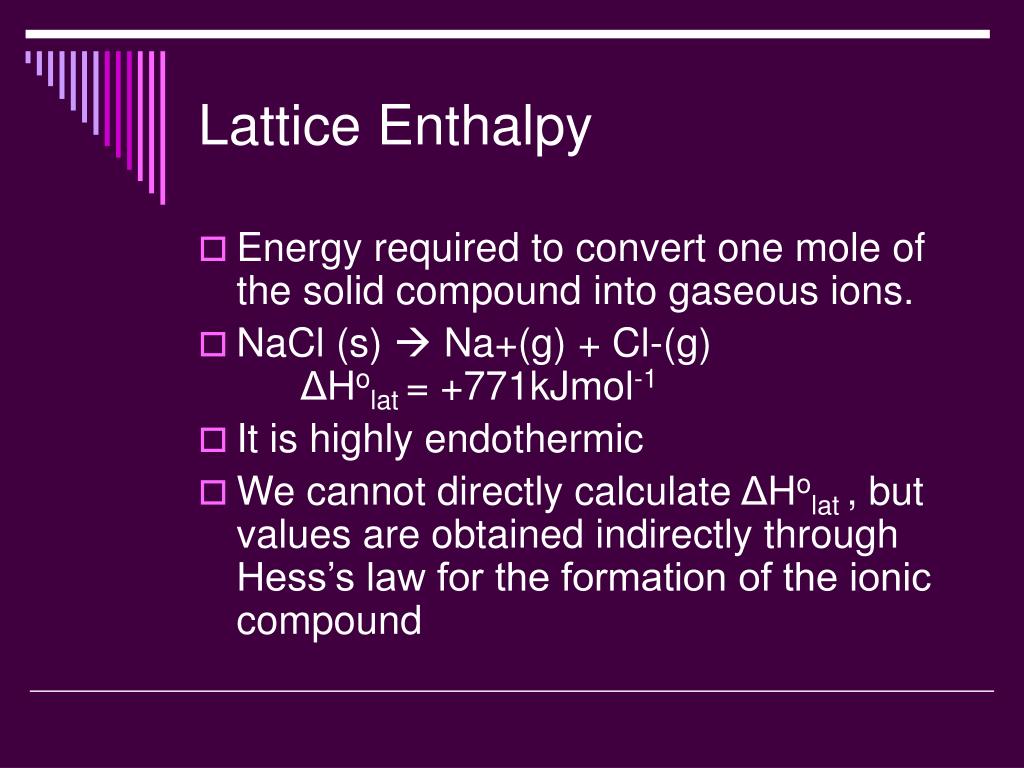
When all other parameters are kept constant, doubling the charge of both the cation and anion quadruples the lattice energy. Thus, the lattice energy of an ionic crystal increases rapidly as the charges of the ions increase and the sizes of the ions decrease. In which C is a constant that depends on the type of crystal structure Z + and Z – are the charges on the ions, and R o is the interionic distance (the sum of the radii of the positive and negative ions). The lattice energy Δ H lattice of an ionic crystal can be expressed by the following equation (derived from Coulomb’s law, governing the forces between electric charges): When one mole each of gaseous Na + and Cl – ions form solid NaCl, 769 kJ of heat is released. Thus, it requires 769 kJ to separate one mole of solid NaCl into gaseous Na + and Cl – ions. For sodium chloride, Δ H lattice = 769 kJ. In both cases, a larger magnitude for lattice energy indicates a more stable ionic compound. Thus, make sure to confirm which definition is used when looking up lattice energies in another reference. Another way is to use an equivalent but opposite convention, wherein the lattice energy is exothermic (negative values) and described as the energy released when ions combine to form a lattice. Here, the convention is used where the ionic solid is separated into ions, meaning the lattice energies will be endothermic (positive values). For the ionic solid sodium chloride, the lattice energy is the enthalpy change of the process: The lattice energy (Δ H lattice) of an ionic compound is defined as the energy required to separate one mole of the solid into its component gaseous ions. The lattice energy of a compound is a measure of the strength of this attraction. Thus, the magnitude of lattice energy is directly proportional to the product of the ion charges and is inversely proportional to the distance between the ions.Īn ionic compound is stable because of the electrostatic attraction between its positive and negative ions. This is why it takes nearly four times as much energy to separate the calcium oxide into gaseous ions as compared to sodium fluoride. However, calcium and oxide ions are divalent and the product of their charge is 4, which is four times greater than sodium fluoride.

The ionic distance in both compounds is similar, but the lattice energy of calcium oxide is almost four times greater than sodium fluoride.īoth sodium and fluoride are monovalent ions the product of their charge is 1. According to Coulomb’s law, the potential energy of ions is directly proportional to the product of their charges.Ĭonsider two ionic compounds sodium fluoride and calcium oxide. In addition to the ionic radius, the magnitude of lattice energy also depends on the ion charges. Thus the lattice energy to separate solid lithium bromide is larger than for potassium bromide being +807 kJ/mol versus +682 kJ/mol, respectively. For example, the bond length of lithium bromide and potassium bromide differs between 217 pm and 282 pm, respectively.ĭue to the increased internuclear distance, the attraction between the ions decreases and it becomes much easier to separate the ions.

As the size of the metal ion increases, the distance between ions, or the bond length, becomes larger as well. In the periodic table, the ionic radius of the alkali and alkaline earth metals increases down the column. Why does each ionic compound have a different lattice energy, and which factors does it depend on?Īn ionic compound consists of an orderly arrangement of a large number of charged ions attracted to each other by electrostatic interactions.Īccording to Coulomb’s law, the potential energy of two ions is inversely proportional to the distance between the ions, which in turn depends on the ionic radius. Yet, when sodium chloride or magnesium oxide form, their lattice energy differs significantly. Lattice energy is associated with the formation or separation of an ionic lattice.


 0 kommentar(er)
0 kommentar(er)
